On January 30, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a "public health emergency of international concern", and then a pandemic on March 11. On January 31, almost 100 science journals and institutes signed a commitment to making data and research on this disease freely available for the duration of the outbreak (the same thing happened early in 2016 regarding Zika). These published articles are open access, and journals also expedite the peer-review process of COVID-19 manuscripts they review. Some prescription-only newspapers like the New York Times are also now making their coronavirus-related coverage available to everybody.
Today (March 12th) Maryland, where we live, has announced schools will close for two weeks starting this Monday the 16th. Different offices/companies/organizations (governmental and private) have been letting employees work from home or have closed the offices and are working remotely.
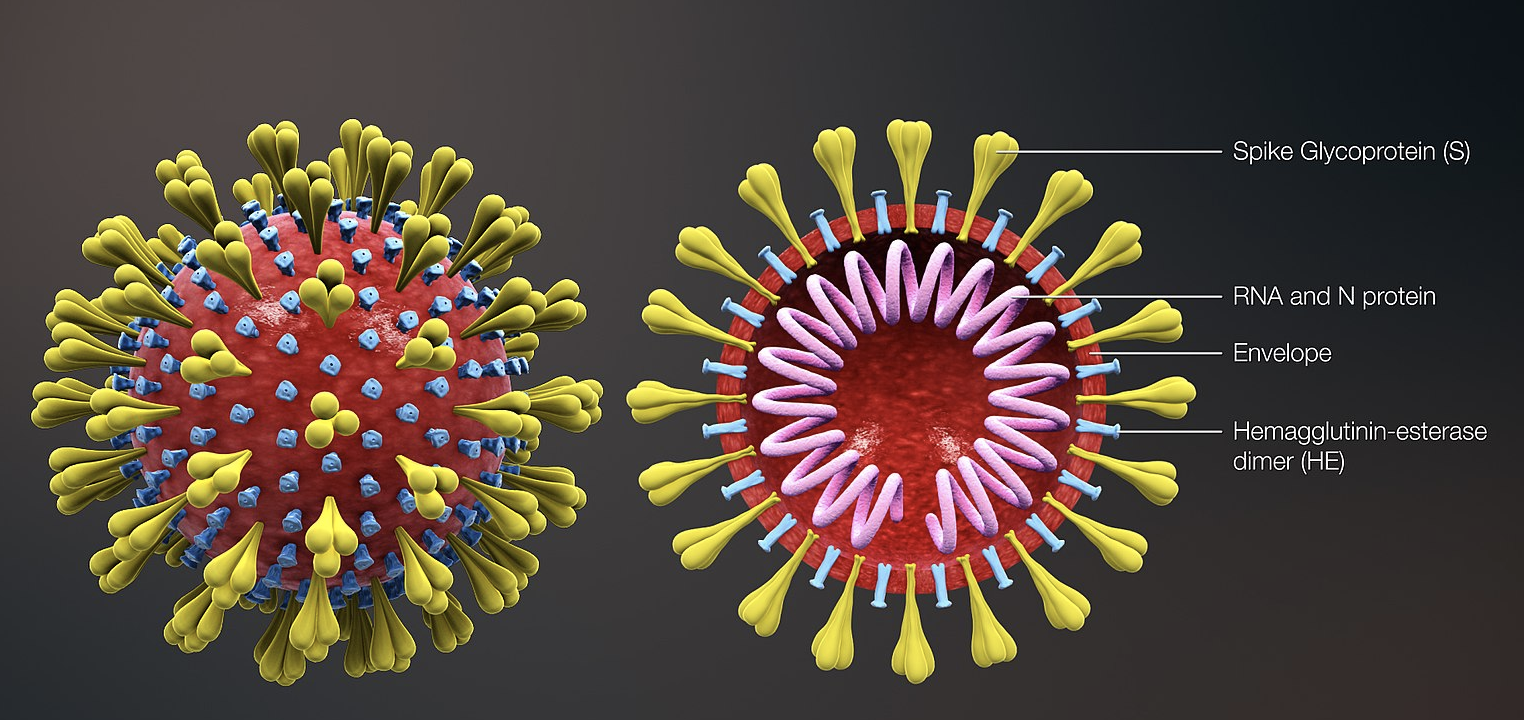
Coronaviruses are enveloped single-stranded RNA viruses of four genera, one of which is the beta-coronaviruses that includes SARS and MERS. SARS-CoV-2, the causative agent of COVID-19 disease, as other coronaviruses, is sensitive to ultraviolet rays and heat and can be effectively inactivated by soap and ethanol (>60% concentration). Transmission is believed to occur via respiratory droplets from coughing and sneezing and by aerosol transmission with elevated aerosol concentrations in close spaces; close contact between individuals seems required. The incubation time is between 2 days and 2 weeks and symptoms include fever, cough and shortness of breath. The "basic reproduction number" or R0 is about 2.2, meaning each infected person transmits the virus in average to 2.2 other people (the R0 of the previous SARS-CoV epidemic was approximately 3).

Because viruses mutate rapidly, sequences of new strains emerging in different location worldwide is important, and is the focus of researchers who make sequences publicly available at international databases such as GenBank. A mutation in an envelope protein-encoding gene in SARS-CoV-2 probably occurred in late November 2019 that triggered jumping to humans from an animal species carrying the virus (see my zoonotic diseases post for information about human diseases of animal origin). Very soon after the first outbreak of COVID-19 was identified in Wuhan, China, the Chinese CDC reported in the journal The Lancet the sequence of the virus causing the disease from samples isolated from 9 patients, which were found to be very similar (about 99%) and 88% related to bat SARS coronaviruses. They were not so close in sequence to the first SARS-CoV (~79%) or MERS-CoV (~50%).
The first SARS as well as the MERS epidemics were contained eventually, we learned a few lessons since, and there has been research on viral mechanisms (including in animal models) and possible vaccines. For example, SARS-CoV and MERS-CoV transmission between humans occurred mainly at hospitals (known as nosocomial transmission), where there were clusters probably due to virus shedding after the onset of symptoms. Family transmission occurred less frequently (13-21% for MERS, 22-39% SARS cases), while transmission between patients was the most common for MERS (62-79% of cases) and to health care workers by infected patients for SARS (33-42% cases).
Based on the viral sequence first released by China, PCR-based molecular tests (see my post on PCR for more info) were developed, which detect viral genetic material (RNA) from patient samples. PCR tests were the ones initially developed/used by the US CDC. China and Singapore have developed serological tests, which detect from blood samples antibodies our bodies produce in response to the viral infection. All these tests were developed quickly in response to the initial outbreak in China as well as subsequent ones in other locations.
These samples are taken mostly from the upper respiratory tract (nasal and oral swabs) but COVID-19 is known to be a lower respiratory tract infection (chest and lungs) and some studies on patients so far show that detection is better from the latter type of samples. As case reports get published and made available immediately, it is clear that testing is critical, for example today a letter reported case of a 69-year-old male in China this past January with pneumonia that was co-infected with SARS-CoV-2 and influenza A, however he was originally misdiagnosed (negative for COVID-19) based on upper respiratory specimens.
Because influenza viruses are seasonal, causing the flu typically to rapidly spread in the fall-winter and decrease in spring and summer (I grew up in a tropical country where there was no flu awareness, or flu shot every year), there has been some speculation that the COVID-19 pandemic may subside in the upcoming months in the summer. But there is no concrete evidence (as stated by WHO) that this is indeed the case. The virus is new to humans, there is no prior immunity and only time and research will tell us more about transmission, spreading, viral mechanisms and possible seasonality. Even if this is the case, with further spreading the virus could reach (and cause more outbreaks in) countries in the southern hemisphere, which are heading towards their winter when we will be in our summer.
Although SARS-CoV-2 can infect people of all ages, current evidence suggests that older people (>60 years of age) and/or those with underlying medical conditions (cardiovascular (heart) disease, diabetes, chronic respiratory disease, cancer) are considerably more susceptible to getting severe disease. Comorbidities were also shown to be important for disease severity in previous SARS and MERS outbreaks, as well as advanced age. These are WHO recommendations for this at-risk population:




 RSS Feed
RSS Feed