In an unprecedented effort to face the pandemic and in addition to national, regional and global initiatives in terms of implementing safety measures such as lockdowns including school and stores closures, mask wearing, PPE and testing, etc, we count now with an impressive number of vaccines approved as well as at different stages of development and in clinical trials. More types of vaccines are being developed simultaneously for COVID-19 than for any other infectious disease before.
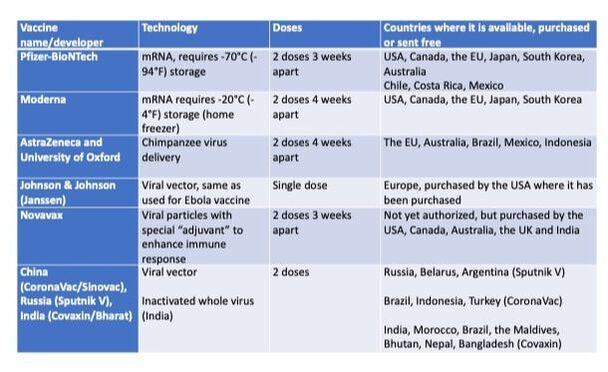
All other vaccines approved to date can be stored at fridge temperatures. These vaccines use different technologies that don’t involve RNA, for example viral vectors are used that have been engineered to carry information on the antigen from the virus, which for COVID-19 is the Spike protein, and to not be able to replicate in human cells. One such vaccine was developed by AstraZeneca and University of Oxford, it got approved by the UK on December 30, 2020. The first registered vaccine was the Russian Sputnik V, in August 2020 before phase 3 clinical trials started, which has been used in Argentina since December 2020. The Janssen (Johnson & Johnson) vaccine is expected to get FDA emergency use approval soon in the USA, the third one after Pfizer and Moderna. This vaccine is the only one so far offering a single dose regimen instead of two.
Because clinical trials are first conducted in adults, COVID-19 vaccines are not yet approved for use in under 16-18 year old children, but trials in younger children are currently underway and a vaccine may become available either by the end of 2021 or in 2022.





 RSS Feed
RSS Feed