These tools, used in “gene editing” systems, are constantly being researched and manipulated to make them more sophisticated, less error-prone and more affordable and efficient to use in cell systems such as human cells and tissues to cure diseases including different types of cancer. We usually hear about these discoveries and applications when they are being used in clinical trials, such as the very recent report of the CRISPR-Cas9 system used in a clinical trial in China where engineered cells were delivered into a patient with aggressive lung cancer. The procedure involved taking specialized immune cells (“T cells”) out of the patient to manipulate them in the laboratory with the CRISPR-Cas9 system to inactivate a gene that normally would prevent immune cells from attacking cancer cells (PD-1 gene). This inactivation may make the immune system more effective in attacking cancer cells. Other diseases with human clinical trials coming up in the near future are sickle cell anemia and beta thalassemia (blood disorders), Huntington’s disease, and cystic fibrosis.
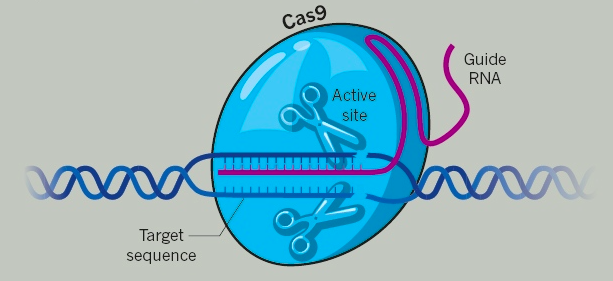
Once the double-strand cut is made in the target DNA by the CRISPR-Cas9 (or similar) system, the cell’s endogenous mechanisms proceed to repair the damaged DNA ends in a process that involves either recession or addition of bases end eventual “ligation” to bind the DNA ends and repair the break. The net result is the introduction of a desired mutation (usually inactivation of the target gene) that will result, hopefully, in the intended effect at the cellular level.
CRISPR has also been used in mosquitoes to lead to an engineered population of Aedes aegypti which can not breed anymore, aiming at stopping transmission of diseases to humans including malaria, dengue and Zika. These mosquitoes have been deployed in preliminary tests in Brazil, Panama and the Cayman Islands, with a net result of almost complete reduction in the mosquito population after 3 million engineered mosquitoes were released. However, an alternative strategy for mosquito elimination has been tested previously with success in Brazil, consisting of releasing Wolchabia bacteria-infected mosquitoes (see my post on Chikungunya for details on this strategy).
CRISPR is also being applied to intense high troughput research approaches. The system has been recently successfully introduced into T cells from donors in a “screen” approach to generate different mutations on cell batches coming from the same donor and then screened for HIV infection. A few mutants were identified as HIV resistant. These methods can be used to investigate the role of genes of interest, screen for drugs that may be effective against specific mutant forms that may be cause of disease, assess the effect of mutations on sensitivity to drugs or infections, and so on.

These impressive technology advances should be recognized as (yet another) development only possible due to the study of those critters we call bacteria. So after thanking yeast in my previous post, on to these precious microorganisms who give us so much (see post on microbiomes for more thanking reasons)
(cartoon from http://freedesignfile.com/173202-funny-cartoon-bacteria-and-virus-vector-10/

 RSS Feed
RSS Feed