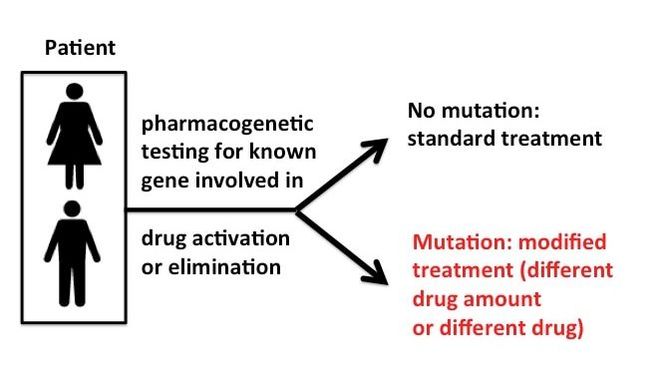
Similarly, the identification of genetic markers for patients’ response to specific drugs is another active research field which focuses on whether or not a patient has a high chance of responding (or not) to a specific treatment, what is the optimal treatment dose, and whether he/she is prone to specific adverse side effects. These predictions, as well as subsequent prescription of appropriate treatment regimen (including drug and drug amount) are based on identified genetic “variants” which are mutations in specific genes in some individuals.
Why should a particular mutation in our DNA affect our chances of a drug therapy working as expected for us? In order for a specific drug (medication) to work inside our bodies at the target tissues/cells, the drug has to be sometimes transported and “metabolized” or activated, meaning it needs to be transformed via biochemical reactions mediated by specific “enzymes” (provided by our cells) in order to have the expected pharmacological effect. After some time, the drug is inactivated or eliminated from our bodies. Mutations in genes that affect metabolism/activation of the drug could lead to treatment failure or reduced efficacy, whereas mutations in genes affecting drug inactivation/elimination may lead to drug accumulation and toxicity and adverse side effects. Mutations in genes that encode the actual targets of a drug also lead to a lack of treatment effectiveness.
These mutations or “genomic variants” (pieces of DNA in our genomes that vary in a population) are now recognized as important factors influencing the outcome of drug treatment. This information is now, as recommended by the FDA, included in labels of some drugs. On the following website there is a table showing FDA-approved drugs with pharmacogenomic information in their labeling: https://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm
For several of these drugs the biomarker is the gene encoding the G6PD enzyme (the most “polymorphic” (variable) gene in humans) for which warnings and precautions are indicated including for the antimalarial drug primaquine- this example is explained in detail in my previous post on malaria.
But in order for pharmacogenetics to be useful in prescribing the right medication and dose of a drug, molecular testing of the patient is required prior. Different tests are available for known mutations that affect the outcome of drug therapies for medical conditions, however these are not always available or applied.

 RSS Feed
RSS Feed